Introduction: Bruton's Tyrosine Kinase (BTK) is an essential component of normal and malignant B-cell receptor signaling. Covalent BTK inhibitors have transformed the treatment of B-cell malignancies. Despite the marked efficacy of covalent BTKi, treatment failure can occur through the development of resistance and discontinuation for adverse events. The activity of these covalent BTK inhibitors are markedly reduced or absent in the presence of BTK cysteine binding site (C481) mutations. Moreover, these agents share pharmacologic liabilities (e.g. low oral bioavailability, short half-life) that may sometimes lead to suboptimal BTK target coverage, for example in rapidly proliferating tumors with high BTK protein turnover, ultimately manifesting as acquired resistance for some patients. To address these limitations, LOXO-305, a highly selective, non-covalent BTKi that inhibits both WT and C481-mutated BTK with equal low nM potency was developed. Proof-of-concept Phase I results demonstrated LOXO-305's anti-tumor activity across patients with heavily pretreated B-cell malignancies (Mato et al. ASH 2019). We previously showed pre-clinical data demonstrating that LOXO-305 potently inhibited wild-type (WT) BTK and different variants of the BTK mutation C481 with nanomolar potency and caused regression in BTK-dependent lymphoma mouse xenograft models (Brandhuber et al. SOHO 2018, and Gomez et al. ASH 2019).
Here we describe the activity of LOXO-305 alone or in combination with venetoclax (BCL-2 inhibitor), in TMD8 BTK WT and TMD8 BTK C481S human tumor xenograft models of diffuse large B lymphoma and a REC-1 human tumor xenograft model of mantle cell lymphoma in mice. We also report the activity of LOXO-305 alone and in combination with R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) or obinutuzumab (anti-CD20 antibody), in the TMD8 xenograft tumor model.
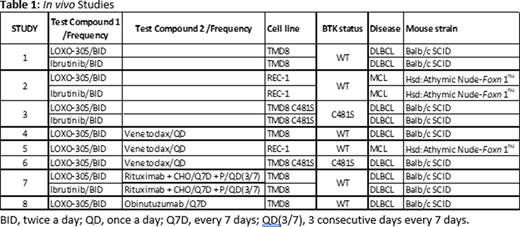
Methods: In all studies, tested articles were administered alone and in combination, following different dosing regimens. Table 1 shows the tested compound(s), dosing frequency, cell line used, disease, BTK status (WT or C481S), and mouse strain used, for each study presented in this abstract.
Human TMD8 BTK WT, TMD8 BTK C481S or REC-1 cells were injected subcutaneously in the right flank of mice. When tumors reached a mean volume between 150 mm3 and 400 mm3, mice were randomized based on their tumor volumes. Mice were next dosed for 17 to 23 days depending on the study design. The potencies of the compounds stand-alone or in combination on the inhibition of the tumor growth were assessed based on the tumor volume changes and weights after collection at the end of the study. Additionally, in the TMD8 studies, the plasma concentrations of tested articles were measured at multiple time points after the last dose.
Results: All treatments were well tolerated without any significant body weight loss or clinical signs being observed on the mice. LOXO-305 potently inhibited the growth of BTK WT and BTK C481S driven xenograft tumors. In all combinations tested, significantly greater tumor growth inhibition was observed in groups where LOXO-305 was co-administered with clinically approved agents.
Conclusion: These data suggest that the co-administration of LOXO-305 with venetoclax, R-CHOP or obinutuzumab could have an increased benefit for patients with B-cell malignancies compared to stand-alone treatments and warrants further investigation.
Gomez:Loxo Oncology, Inc, a wholly owned subsidiary of Eli Lilly and Company: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months. Wu:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Stephens:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Rosendahl:Loxo Oncology, Inc, a wholly owned subsidiary of Eli Lilly and Company: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months. Brandhuber:Loxo Oncology, Inc, a wholly owned subsidiary of Eli Lilly and Company: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal